
How Intermittent Fasting and Exercise Affect Muscles and Fat: New Clues from Metabolic Research in Mice
As obesity and type 2 diabetes continue to rise globally, understanding how lifestyle interventions affect the body at a biological level is increasingly important – particularly in children with obesity as they face a higher risk of developing type 2 diabetes later in life.
In a recent study conducted by our partners at the DIfE, Heike Vogel, Annette Schürmann et al. explored how lifestyle behaviours like diet and exercise influence gene regulation through a process called alternative splicing – a key step to regulate gene expression profiles – in the muscle and fat tissues of mice.
While the health benefits of these behaviours are well known, the role of alternative splicing in metabolic improvements related to lifestyle remains largely unexplored, despite its implication to diseases like obesity and type 2 diabetes if the process becomes dysregulated.
The Obelisk project applies a 4 P’s approach to obesity - prevention, precision, participation and prediction – to identify effective interventions that can support healthier outcomes from the earliest stages of life. This research supports that aim by exploring cellular mechanisms that may influence metabolic health, helping to inform science-based strategies for predicting and preventing childhood obesity and its related comorbidities.
This article explains the study’s key findings and their potential implications for future human research into type 2 diabetes prevention.
Understanding Alternative Splicing
Our genes serve as a blueprint or set of instructions for producing proteins, which are responsible for nearly all functions in the body.When a cell needs to produce a protein, it first copies the gene from DNA into a temporary message called RNA. This RNA often includes extra segments or optional instructions.Alternative splicing makes variations of that message, allowing cells to produce different versions of proteins from the same gene based on what the cell needs at that time.
This process is crucial for creating protein diversity and adapting to various conditions, even small tweaks in protein versions can affect how well our bodies handle things like blood sugar, fat storage and energy.
When there are errors in the splicing process, it can lead to diseases like type 2 diabetes. The research conducted by our partners at DIfE aimed to uncover how intermittent fasting, and exercise might correct these splicing errors and improve insulin resistance.
What did the Researchers Study?
Researchers wanted to see if lifestyle changes like fasting or exercise could influence alternative splicing in two key areas:
- Skeletal muscle, where we burn most of our energy
- White fat tissue, where we store energy
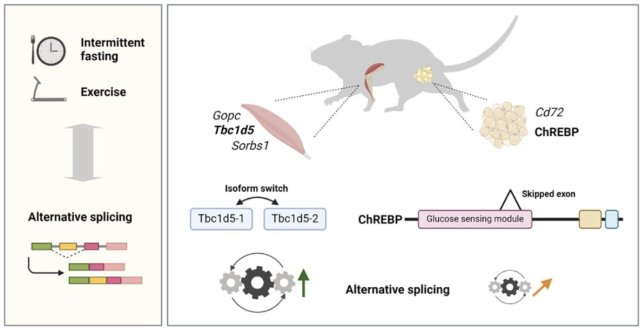
The researchers used two groups of mice:
- NZO mice; which are susceptible to obesity and type 2 diabetes
- Healthy C57BL/6J mice; used as controls
The mice then underwent different lifestyle interventions:
- Intermittent fasting: The NZO mice were either fed every other day (alternate-day fasting) or had restricted feeding times (time-restricted feeding- eating within an 8-hour window).
- Exercise: The healthy mice were trained on a treadmill.
They then examined the muscle and fat tissue to see whether these interventions affected alternative gene splicing.
Key Findings from the Study
Muscle vs. Fat Tissue Response:
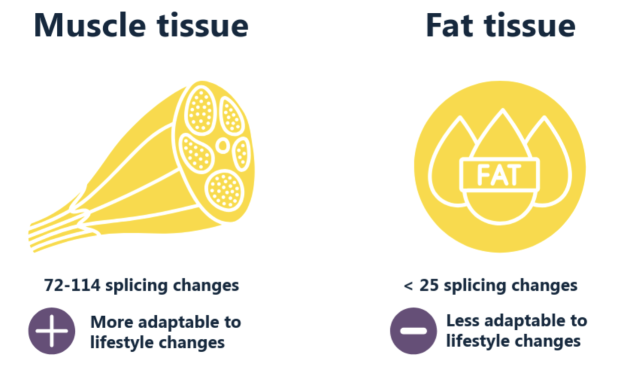
Muscle tissue showed more flexibility in gene splicing compared to fat tissue. There were 72-114 splicing changes in muscle, while fat tissue had fewer than 25 changes. This suggests that muscle tissue is more responsive to lifestyle changes.
Impact of Intermittent Fasting:
Both types of intermittent fasting (alternate-day and time-restricted) led to changes in gene splicing, but these changes were generally mild.
Some of the genes affected are involved in key metabolic processes like glucose sensing and insulin signalling. For example, the data showed that intermittent fasting caused changes in splicing in skeletal muscle, affecting genes like Sorbs1, Raf1, and Gopc, which are involved in insulin signalling and helping glucose enter cells, and alternate day fasting affected the splicing of a gene called Mlxipl, which is involved in glucose sensing and is notably associated with type 2 diabetes in humans, hinting at potential metabolic benefits through splicing.
Exercise Effects:
Exercise also triggered small splicing changes in muscle tissue. One noteworthy change was in the gene Kat6a, which is linked to transcriptional regulation - the process by which cells control which genes are ‘turned on’, influencing the amount and types of proteins produced. These changes highlight the potential of exercise to influence gene expression and how this may influence how muscles respond to physical activity at the molecular level.
Implications for Prevention and Healthcare
These results suggest that, in mice, fasting and exercise can subtly alter how genes are processed in tissues central to metabolism. While the effects aren’t dramatic, they might still help fine-tune metabolism and insulin sensitivity.
By identifying specific genes and splicing patterns influenced by diet and exercise, researchers could potentially target these areas for interventions in the future.
Looking Ahead: The Need for Further Research
This research offers some important observations that may be of interest for future human research trials. By identifying specific genes and splicing events that respond to lifestyle in mice, it lays the groundwork for future studies in humans.
Conclusion
The study provides valuable insights into the molecular effects of intermittent fasting and exercise on muscle and fat tissues in mice. While the changes in gene splicing were generally mild, they highlight the adaptability of muscle tissue and the potential metabolic benefits of these lifestyle interventions. While the effects in humans remain to be confirmed, the study points to promising directions for future research on metabolism and diabetes.
Obelisk aims to develop more personalised prevention and treatment strategies, which could eventually benefit from understanding these kinds of individual-level responses to lifestyle.
As Obelisk continues to explore the complex relationship between genes and lifestyle, particularly the molecular mechanisms that drive or prevent the shift from healthy weight to obesity during early life and associated factors that interact with this process, this study contributes valuable knowledge that could help shape more effective health strategies of the future.
Paper
Jasmin Gaugel, Markus Jähnert, Alexander Neumann, Florian Heyd, Annette Schürmann, Heike Vogel. Alternative splicing landscape in mouse skeletal muscle and adipose tissue: Effects of intermittent fasting and exercise,The Journal of Nutritional Biochemistry, Volume 137, 2025, 109837.